Structure Info
- Chemspace ID
- CSCS06256042981 (Synthesis)
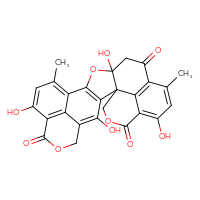
- IUPAC Name
- 3,9,15,21-tetrahydroxy-11,19-dimethyl-6,14,24-trioxaheptacyclo[16.7.1.1⁴,⁸.0¹,¹⁵.0²,¹³.0²²,²⁶.0¹²,²⁷]heptacosa-2(13),3,8(27),9,11,18(26),19,21-octaene-7,17,23-trione
- Mol formula
- C26H18O10
- Mol weight
- 490 Da
- Catalog Number(s)
- BDA70663, HY-126649, T37549
- Copy structure to query editor
- SMILES
- INCHI
- INCHI key
- MOL
Properties
- LogP
- 4.1
- Heavy atoms count
- 36
- Rotatable bond count
- 0
- Number of rings
- 7
- Carbon bond saturation, Fsp3
- 0.269
- Polar surface area (Å)
- 160
- Hydrogen bond acceptors count
- 8
- Hydrogen bond donors count
- 4
- Zoom the structure
- CSCS06256042981
Items Overall 2 items from 2 suppliers
| Supplier | Lead time | Ships from | Purity | Pack | Price, $ | Qty |
|---|---|---|---|---|---|---|
| MedChemExpress | 30 days | United States To: | 90 | 1 mg | 471 | |
Description: Names: Bacillosporin C; Product Description: Bacillosporin C is an oxaphenalenone dimer originally isolated from T. bacillosporus. Bacillosporin C, an anhydride, is formed from the lactone bacillosporin D in the mangrove endophytic fungus SBE-14. Similar oxaphenalenone dimers have antibiotic activity and inhibit acetylcholinesterase.; Target: Antibiotic; CAS: 76706-63-3 | ||||||
| MedChemExpress EU | 30 days | Sweden To: | 90 | 1 mg | 497 | |
Description: Names: Bacillosporin C; Product Description: Bacillosporin C is an oxaphenalenone dimer originally isolated from T. bacillosporus. Bacillosporin C, an anhydride, is formed from the lactone bacillosporin D in the mangrove endophytic fungus SBE-14. Similar oxaphenalenone dimers have antibiotic activity and inhibit acetylcholinesterase.; Target: Antibiotic; CAS: 76706-63-3 | ||||||
For a custom pack size or bulk
please drop us a line:Enquire
please drop us a line:Enquire