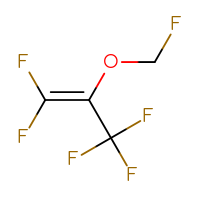
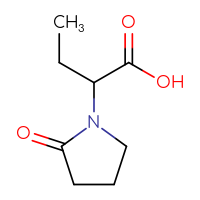
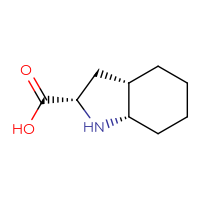
Drug impurities are undesirable compounds that accompany the main drug. They are the result of side processes that are inevitable in large-scale organic synthesis.
It is very important to collect data on the impurities that drugs may have to control the amount of these side substances in the final substance. These impurities are often very hard to remove and are a subject to a drug quality control. For synthetic drugs, thresholds for the number of impurities are usually available.
Chemspace offers a set of the known drug impurities that could be used as references for quality control purposes.
The compounds have annotations from either US Pharmacopeia or European Pharmacopeia.
Ordering options: full set or cherry-picked selection.
For any questions contact us at [email protected]