About GPCRs
GPCRs have been of long-standing interest as pharmacological targets, as they regulate numerous diverse physiological processes and have druggable sites accessible at the cell surface. According to the analysis provided in the review*, GPCRs display the steepest upward trend in protein family popularity in absolute numbers over 20 years since the end of 1990’s.
General description of process
Chemspace computational services team prepared structurally diverse GPCR Targeted Library based on USRCAT similarity. For a better understanding of the approach, we created a description available for downloading. It gives brief information on GPCRs, USRCAT, the algorithm and its validation, explains the process of creating the library.
Some words about USRCAT and how it works
USRCAT (The Ultrafast Shape Recognition with CREDO Atom Types) is a Ligand-Based Virtual Screening (LBVS) method**. This method is capable of retrieving compounds with sharing 3D molecular shape with minimal topological similarity. This means the possibility of finding compounds, that will be completely different by structure but have high potential interaction with the corresponding receptor.
USRCAT condenses 3-dimensional information about molecular shape, as well as other properties, into a small set of numeric descriptors. This gives the ability to compare molecules fast and accurately.
What we offer
As a result of applying steps indicated in the workflow to a set of active compounds from the
GPCRdb classifier, we prepared a set of screening compounds from the Chemspace catalog.
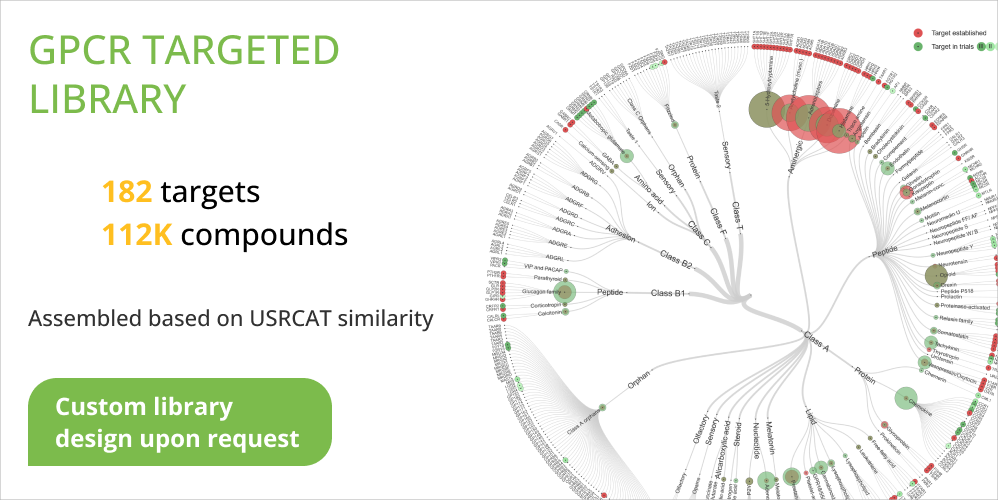
112K of compounds that are potent to show activity against 182 targets belonging to 65 GPCRs families were selected to GPCR Targeted Library by our computational team.
Custom libraries design
If you did not find your target and the targeted library for it, please feel free to request library generation specifically for your target.
You can find the full list of targets in the attachment to the GPCR Targeted Library.
We offer generation targeted libraries using known ligands and analog similarity search by USRCAT (method to search for new chemotypes), as well as methods with Morgan FP and E3FP.
To find out more or request a custom library drop an e-mail at: [email protected].
* Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schiöth, H. B., & Gloriam, D. E. (2017). Trends in GPCR drug discovery: new agents, targets and indications. Nature Reviews Drug Discovery, 16(12), 829–842. doi:10.1038/nrd.2017.178
** Schreyer, A. M., & Blundell, T. (2012). USRCAT: real-time ultrafast shape recognition with pharmacophoric constraints. Journal of cheminformatics, 4(1), 27. doi:10.1186/1758-2946-4-27