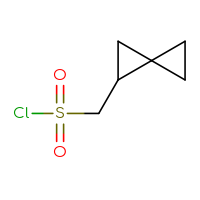
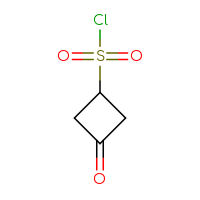
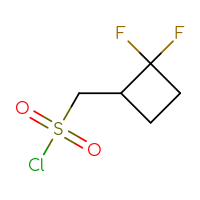
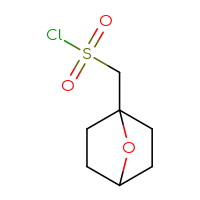
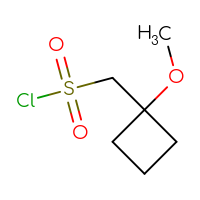
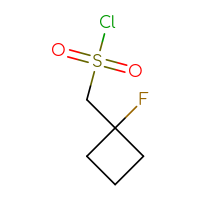
It is an interesting fact that the well-known class of sulfonyl chlorides is still one of the most wanted Building Blocks for many researchers. Is it because of a highly reactive sulfonyl chloride moiety that reacts with anything you want and can rapidly produce the corresponding sulfonic acids, sulfonic esters, or sulfonamides? Or is it because of the sulfone sulfur in a future hit as a huge polar group to make a compound more soluble? Or maybe both reasons? Who knows but the researcher… The only thing that we know for sure is that you, our dear users, simply love sulfonyl chlorides and, at the very least, will be happy just to have a look at the set we have prepared.
Sulfonyl Chlorides. Part 2: https://chem-space.com/news/29
Sulfonyl Chlorides. Part 3: https://chem-space.com/news/32