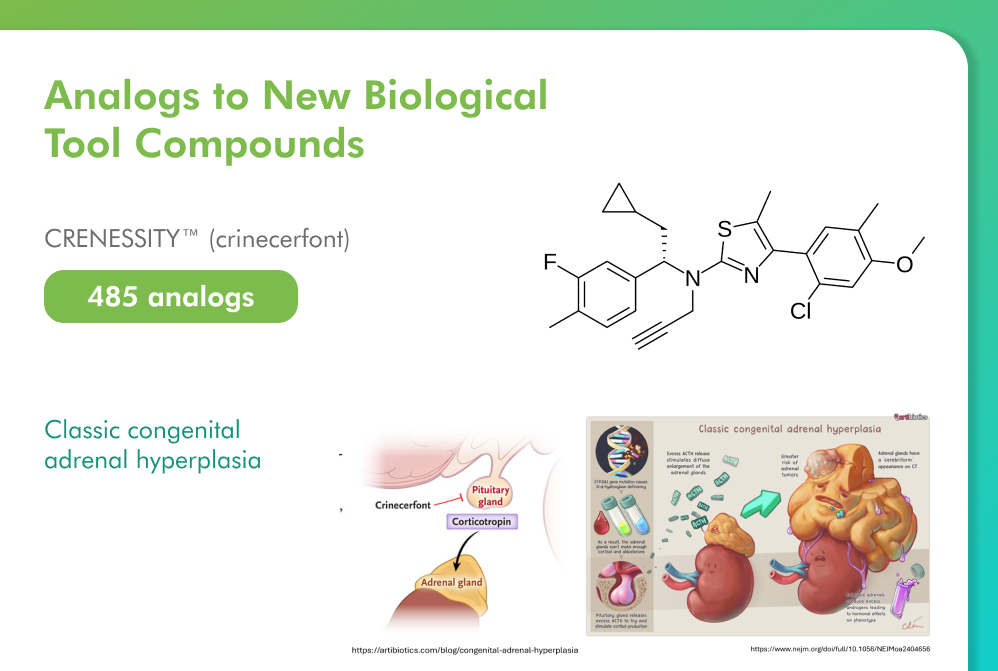
CRENESSITY™ (crinecerfont), the oral antagonist of the corticotropin-releasing factor type 1 receptor (CRF1), was approved by the FDA in December 2024 as the first new therapy in 70 years for classic congenital adrenal hyperplasia (CAH) and represents a significant breakthrough in managing this rare genetic condition.
To further study the therapeutic potential of CRF1 receptor antagonism, we have created a set of analogs for сrinecerfont. (https://chem-space.com/list/c29993334ccc4896895d9a5958a2c9e9)
CRF1 receptors are pivotal in regulating the hypothalamic-pituitary-adrenal (HPA) axis, which is dysregulated in CAH. By inhibiting CRF1, сrinecerfont reduces excessive adrenocorticotropic hormone secretion, offering a non-glucocorticoid mechanism that can reduce the side effects of traditional high-dose steroid therapy. Clinical trials have demonstrated significant reductions in adrenal androgens and androgen precursors in both adult and adolescent patients with classic CAH.
We invite collaboration to study these promising crinecerfont analogs. For orders and inquiries, please get in touch with us at [email protected].