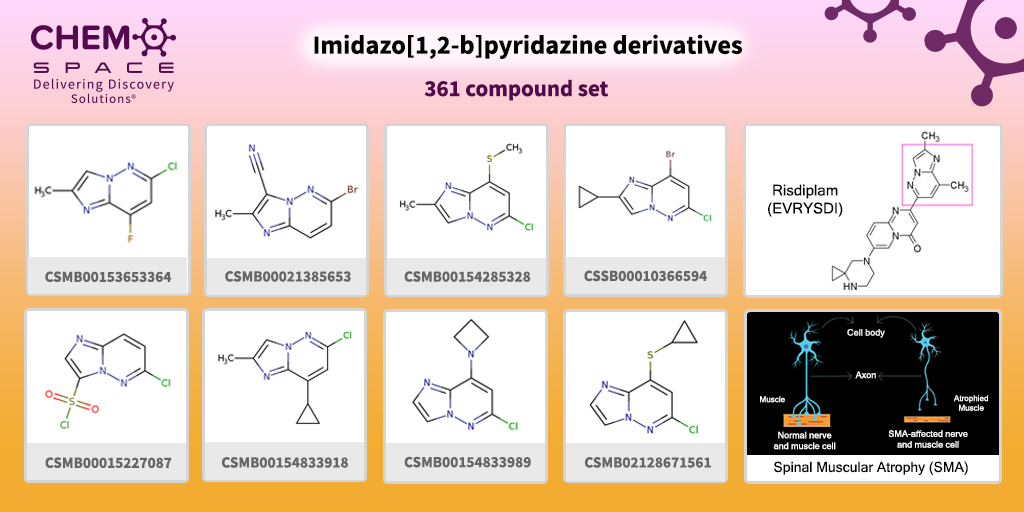
In August 2020, Risdiplam (EVRYSDI) received approval by FDA for the treatment of adults and children with spinal muscular atrophy (SMA). It is a highly potent, selective, and orally active small molecule drug that works by increasing the amount of functional survival motor neuron (SMN) protein produced by the SMN2 gene through modification of the splicing of SMN2 messenger RNA. Risdiplam contains imidazo[1,2-b]pyridazine moiety in its backbone.
We have made a set of Building Blocks, which contain such part – the perfect start for your research!