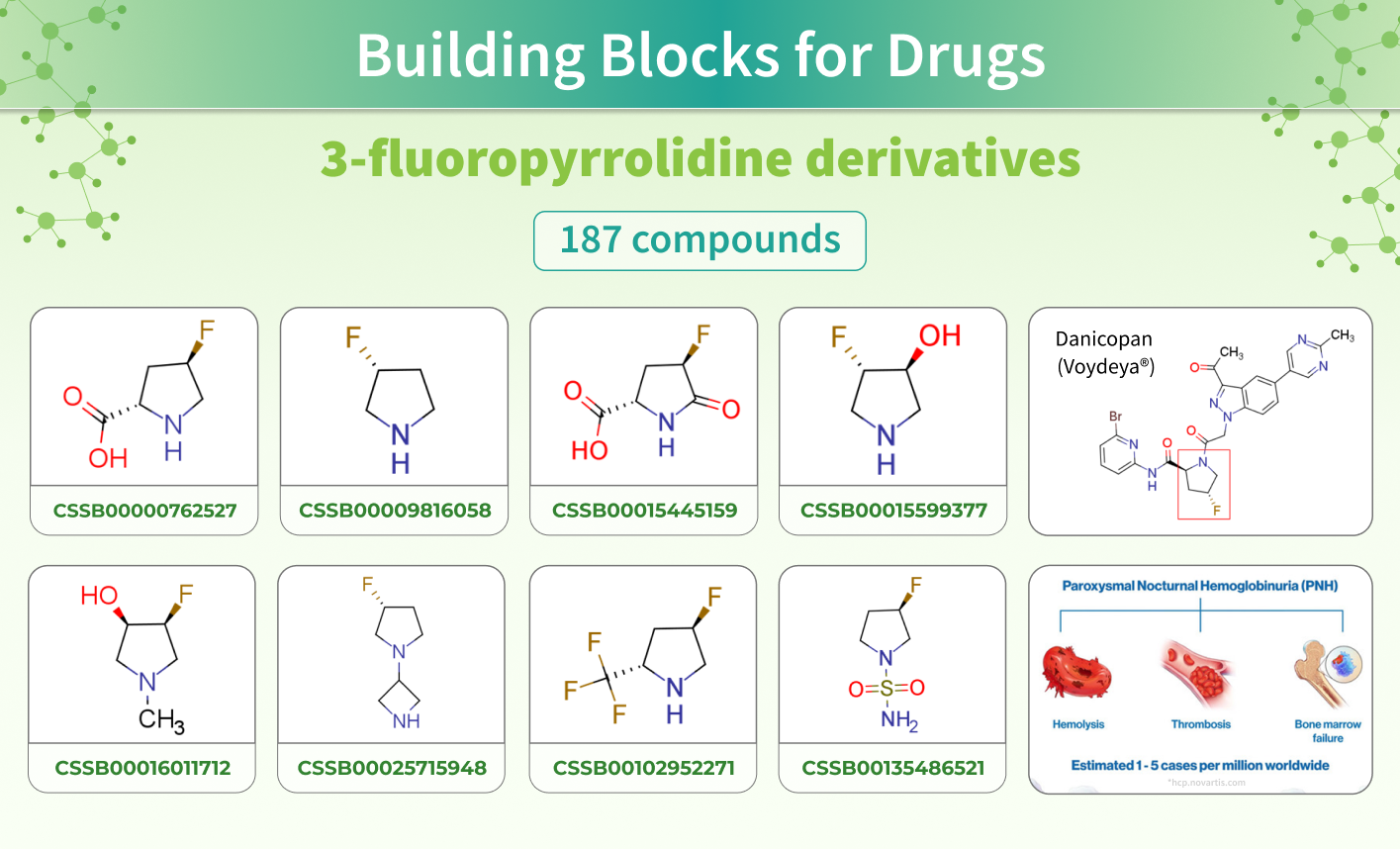
This March, Danicopan (Voydeya®) received FDA approval as a drug for the treatment of extravascular hemolysis with paroxysmal nocturnal hemoglobinuria (PNH). PNH is a rare, chronic, progressive, and potentially life-threatening blood disorder.
This drug's backbone includes a 3-fluoropyrrolidine fragment. To encourage future research, we have developed a set of Building Blocks with this structure at its core, which can serve as a viable beginning point for your research initiatives!
Danicopan, is a medication used for the treatment of PNH. It is a complement inhibitor that reversibly binds to factor D to prevent alternative pathway-mediated hemolysis and deposition of complement C3 proteins on red blood cells.