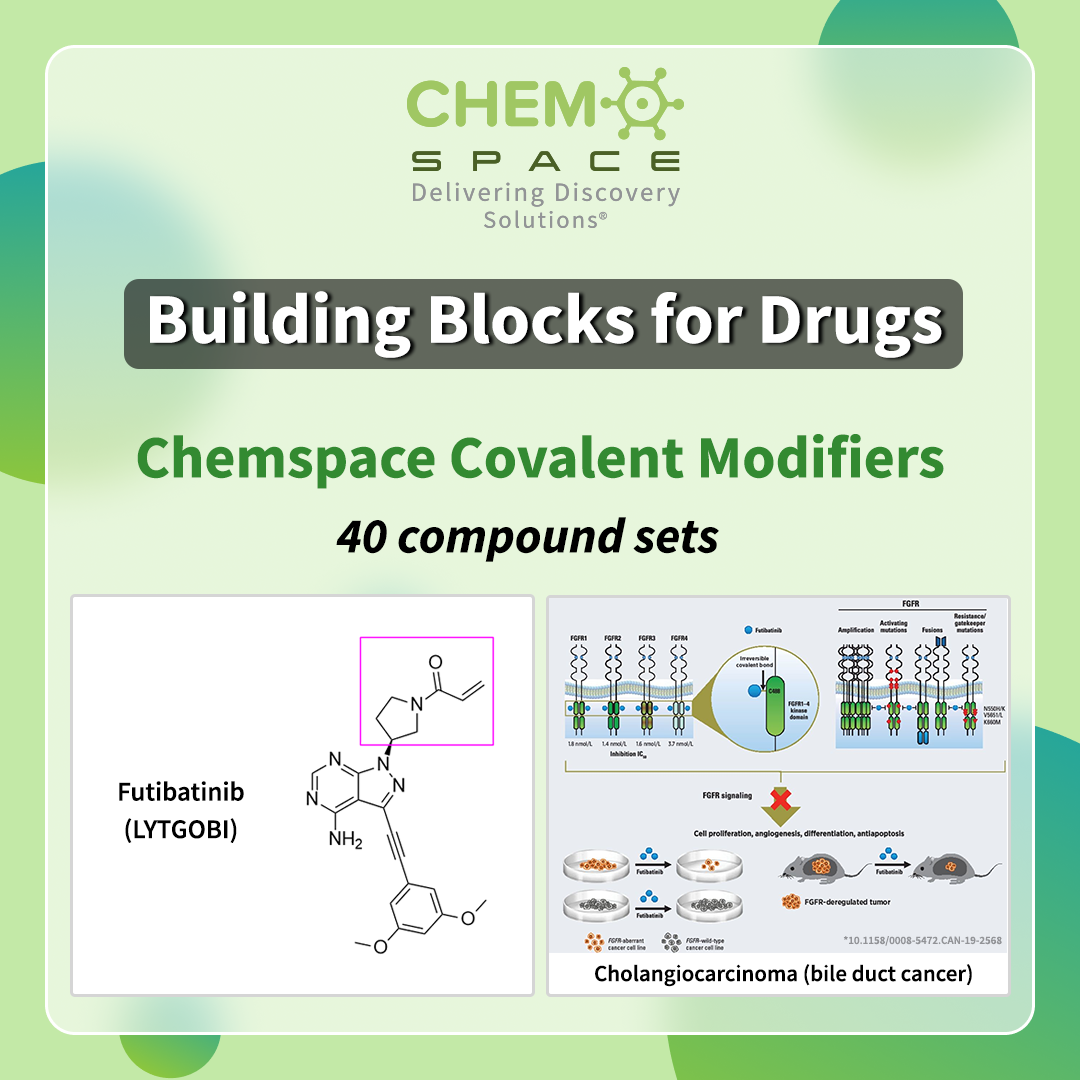
This September Futibatinib (LYTGOBI) received approval by the FDA as an oral drug for the treatment of cholangiocarcinoma (bile duct cancer).
It is a highly specific, potent, irreversible FGFR1–4 inhibitor which shows selective activity against tumors harboring various FGFR aberrations. Futibatinib has a warhead that unalterably binds to the target.
We offer you 40 Covalent Modifiers sets with versatile binders, which could be the perfect start for your research in anti-cancer medications and other fields!