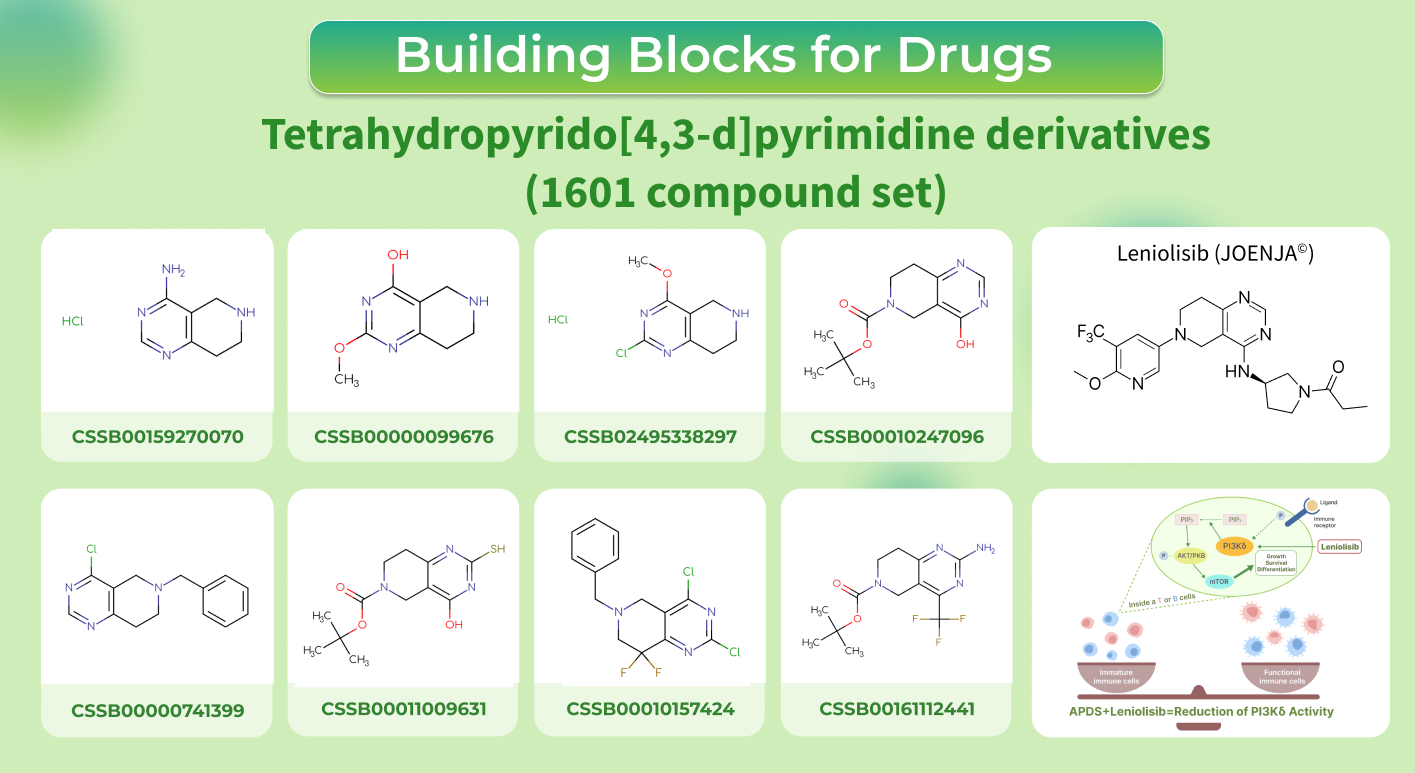
This March Leniolisib (JOENJA©) was granted FDA approval as the first and only medication used for the treatment of activated phosphoinositide 3-kinase delta syndrome (APDS) in patients older than 12 years.
APDS is caused by mutations in PIK3CD or PIK3R1 genes (one or both), that regulate the maturation of white blood cells. As a result, people with APDS experience a decrease in amount of immune cells, making it difficult for them to fight off bacterial and viral infections.
Leniolisib is an oral, potent, and selective inhibitor of phosphoinositide 3-kinase delta (PI3Kδ) protein, which is overactive in APDS. By inhibiting PI3Kδ, Leniolisib helps normalize immune function by significantly increasing the number of immune responses generating B cells and reducing the size of lymph nodes.