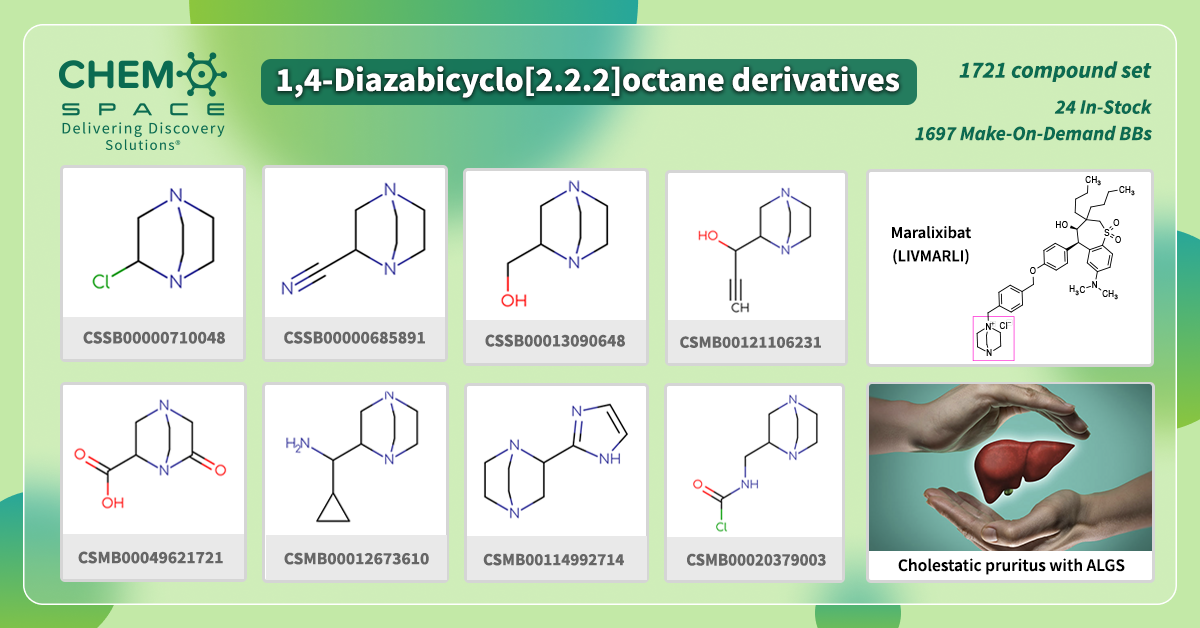
Last September, Maralixibat (LIVMARLI) received approval by the FDA for the treatment of cholestatic pruritus associated with Alagille syndrome.
It is an oral solution suitable for one year of age and older patients within one dose per day. Maralixibat is a reversible inhibitor of ileal bile acid transporter (IBAT) with minimal absorption.
It contains 1,4-diazabicyclo[2.2.2]octane moiety in its backbone.
We have made a set of Building Blocks, which contain such parts – the perfect start for your research!