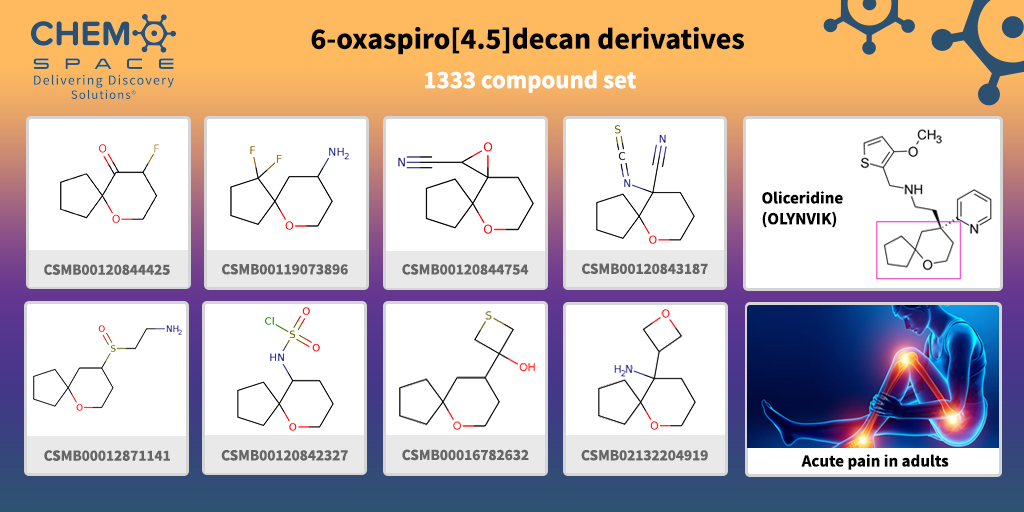
In August 2020, Oliceridine (OLYNVIK) received approval by the FDA for the treatment of acute severe pain in adults. It is an opioid intravenous drug that is a functionally selective μ-opioid receptor agonist.
Oliceridine educes robust G protein signaling, with potency and efficacy similar to morphine, but with far less β-arrestin 2 recruitment and receptor internalization and it displays less adverse effects than morphine.
Oliceridine contains 6-oxaspiro[4.5]decan moiety in its backbone. We have made a set of Building Blocks, which contain such part – the perfect start for your research!