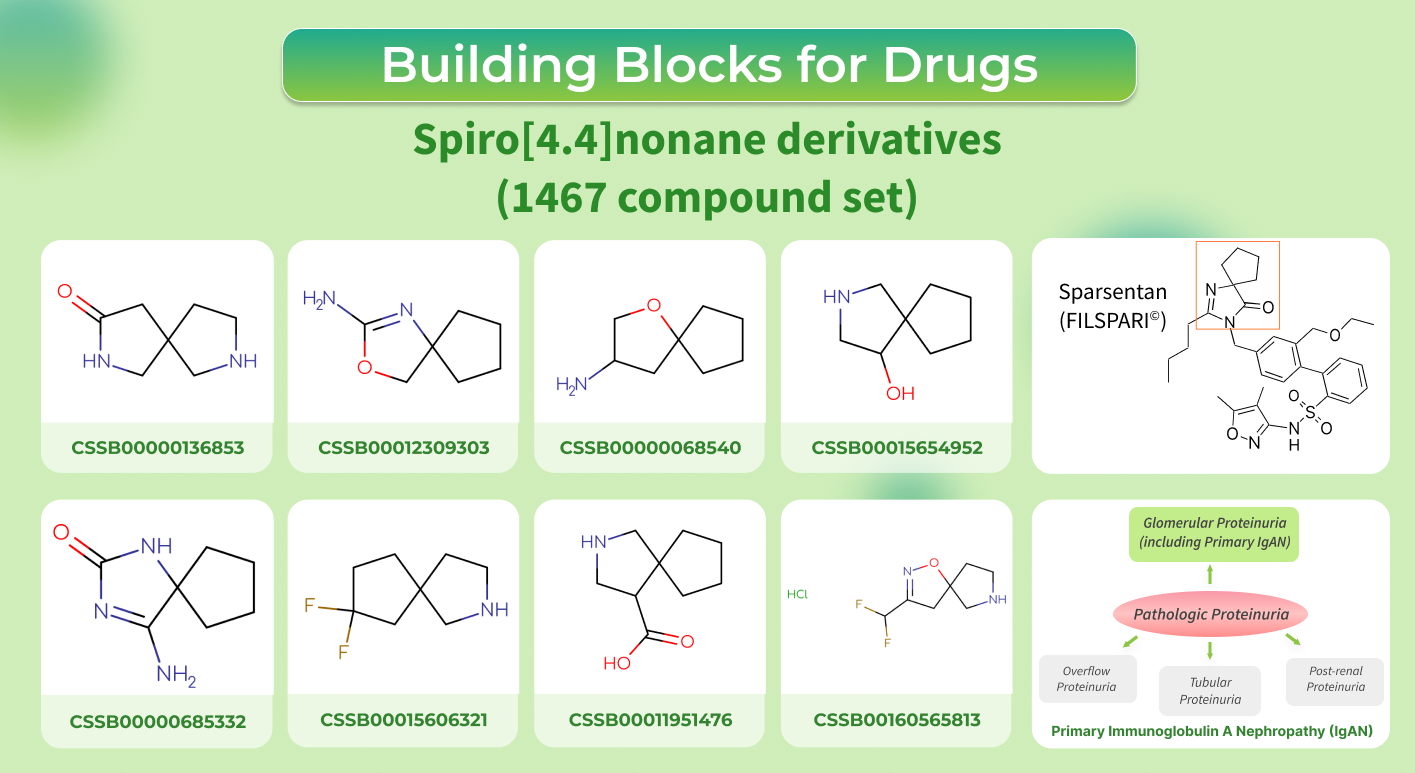
This February Sparsentan (FILSPARI©) received accelerated approval by the FDA as a medicine for treating proteinuria in adults with primary immunoglobulin A nephropathy (IgAN).
This is the first example of non-immunosuppressive therapy against such rare pathology as IgAN. Sparsentan works by blocking two types of receptors - endothelin type A (ETA) and angiotensin II subtype 1 (AT1), which are linked with the progression of kidney disease.