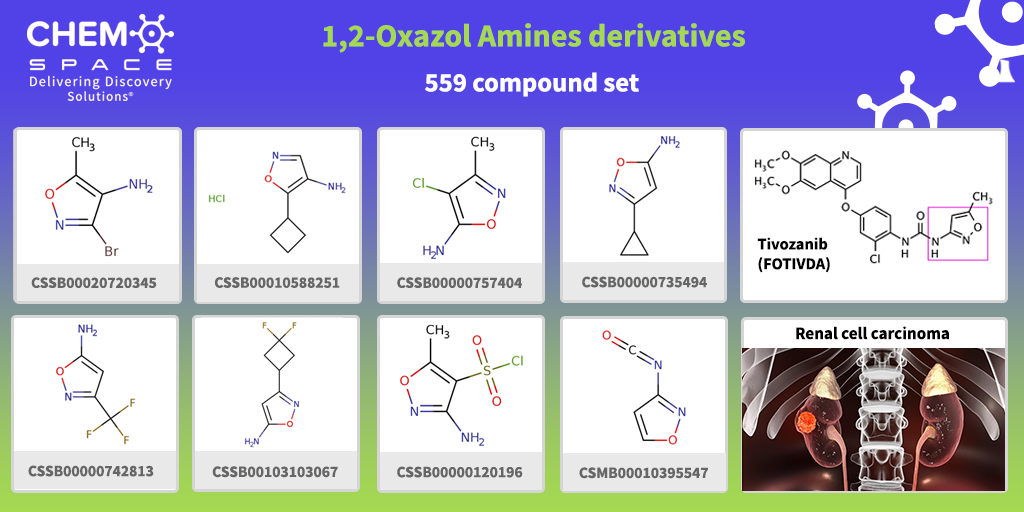
Tivozanib (FOTIVDA) received approval by the FDA in March 2021 as a drug for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma (RCC).
It is an oral, differentiated vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) with QD-dosing.
Tivozanib contains 1,2-oxazole moiety in its backbone.
We have made a set of Building Blocks, which contain such parts with an amine group in different positions – the perfect start for your research!