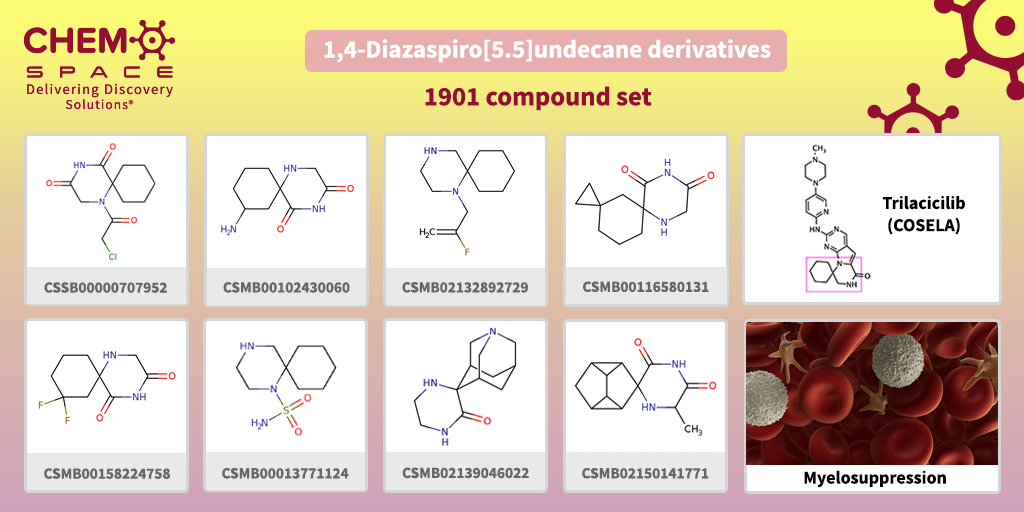
This February, Trilacicilib (COSELA) received approval by the FDA as the first therapy in its class to reduce the frequency of chemotherapy-induced bone marrow suppression in adults receiving certain types of chemotherapy for extensive-stage small cell lung cancer (SCLC).
Trilacicilib is a cyclin-dependent kinase (CDK) 4/6 inhibitor.
It contains diazaspiro[5.5]undecan-3-one moiety in its backbone.
We have made a set of Building Blocks, which contain similar parts – the perfect start for your research!