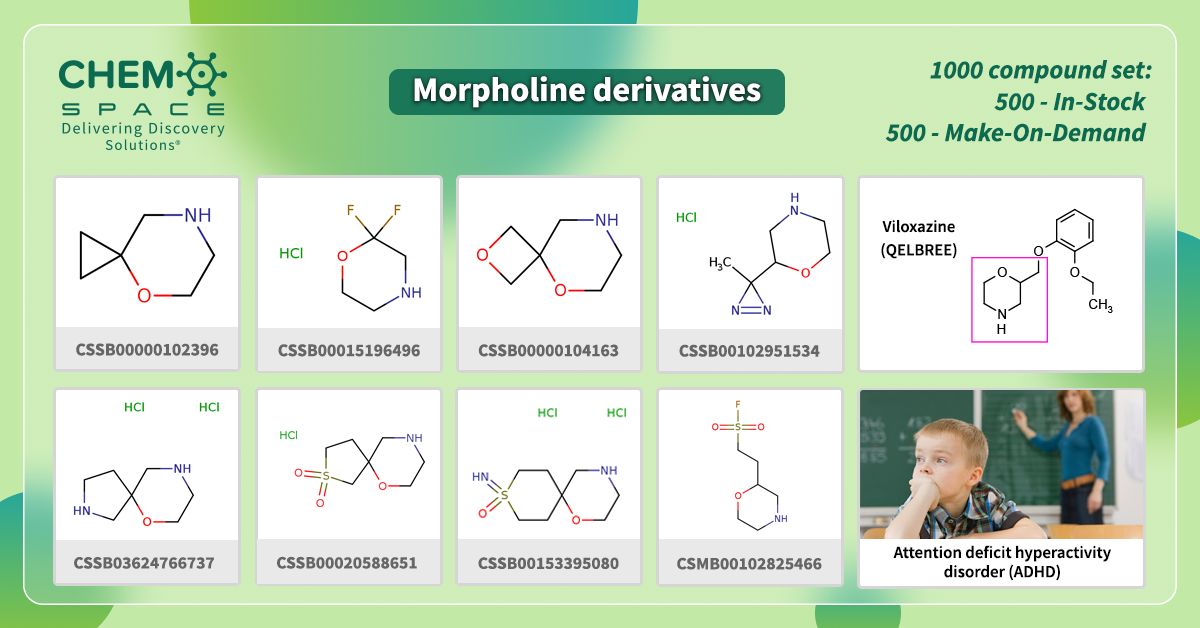
This April, Viloxazine received approval by the FDA as an oral drug for the treatment of attention deficit hyperactivity disorder (ADHD) in children.
It is an example of drug repurposing that was marketed about 20 years ago in some European countries as an antidepressant. Viloxazine acts as a selective norepinephrine reuptake inhibitor (NRI).
It contains morpholine moiety in its backbone.
We have made a set of Building Blocks, which contain such parts – the perfect start for your research!