October is Breast Cancer Awareness Month worldwide. We join the global community in highlighting the importance of early detection, diagnosis, and control management of this dangerous disease.
In October 2024, the FDA approved a new drug, inavolisib, in combination with palbociclib and fulvestrant, for the treatment of specific advanced breast cancer cases.
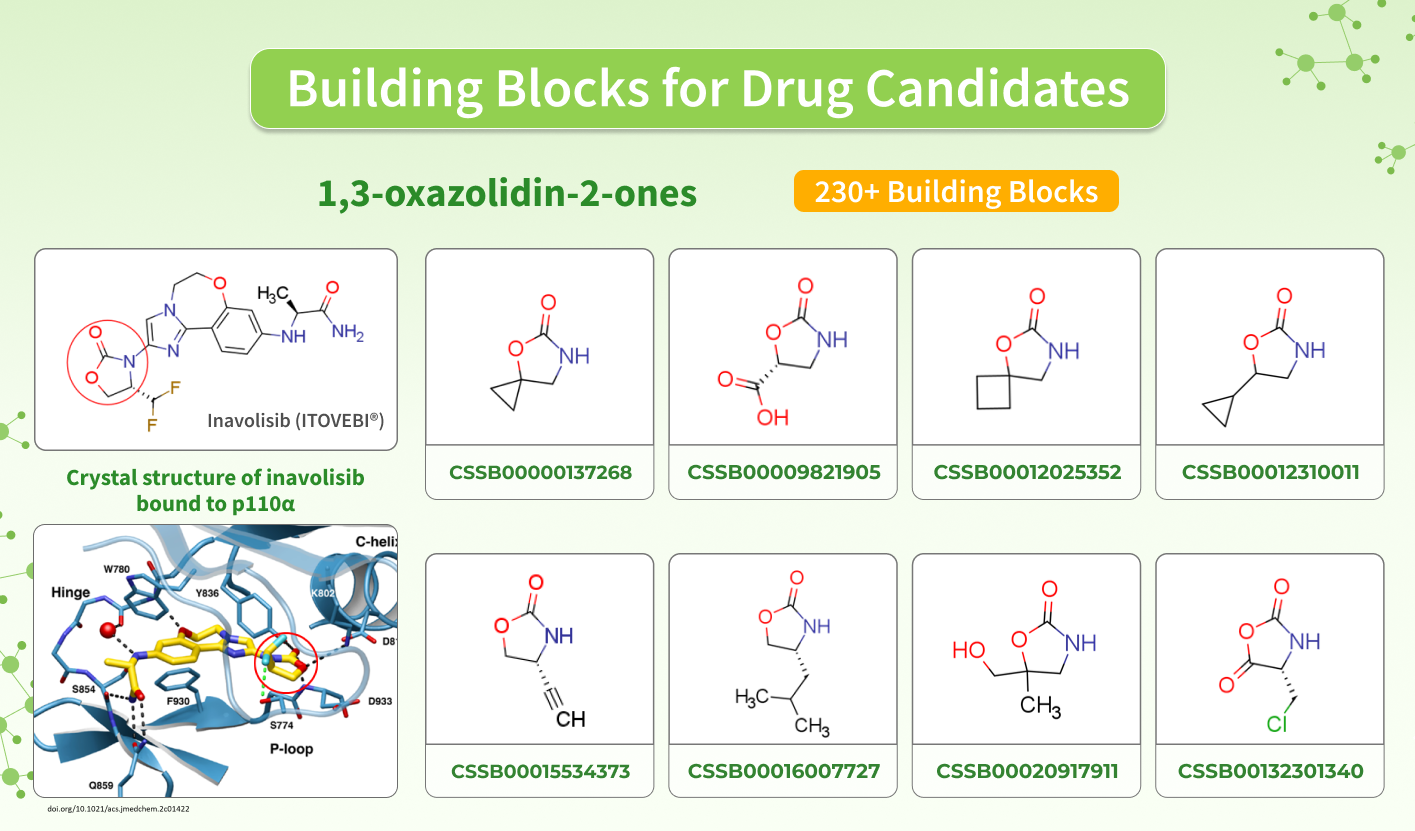
Inavolisib contains a 1,3-oxazolidin-2-one fragment in its structure, which is found in other compounds that exhibit biological activity toward various targets, involved in the development of different pathologies. This is why we offer a set of in-stock and MADE building blocks that you can use to design promising drug candidates.
Inavolisib (Itovebi, Genentech, Inc.) is a selective PI3K-alpha inhibitor used in combination therapy for adults with endocrine-resistant, PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer.