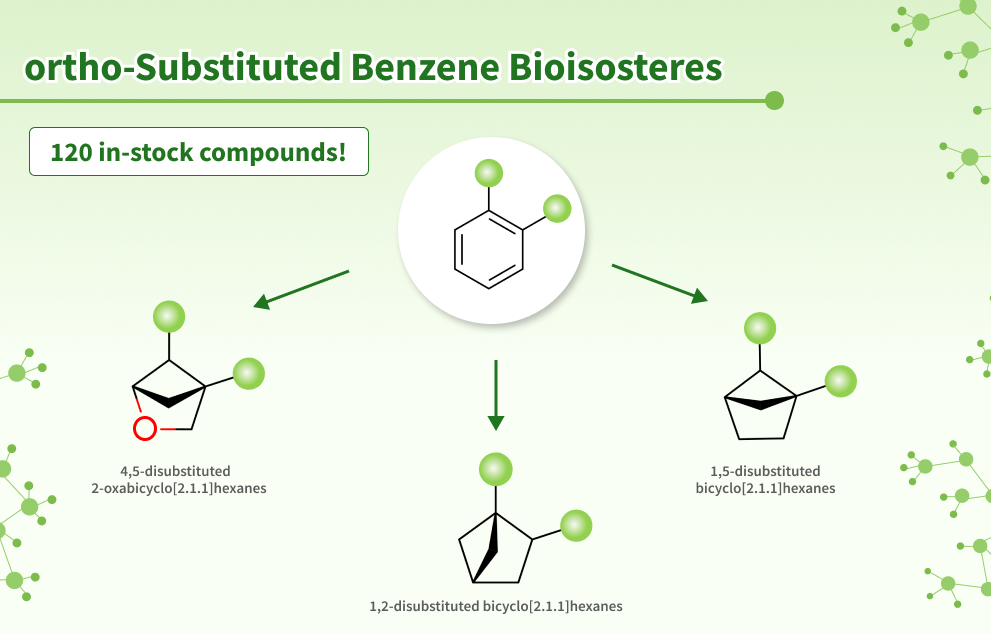
Discover Chemspace ortho-Substituted Benzene Bioisosteres – a library of 120 in-stock compounds featuring saturated bicyclic scaffolds that can potentially mimic 1,2-disubstituted phenyl ring properties. Replacement with these moieties represents a promising tactic leading to enhanced water solubility and decreased lipophilicity with retained biological activity.
Over three hundred approved drugs and agrochemicals contain ortho-substituted benzene rings. However, the presence of several phenyl rings in a molecule can lead to undesirable properties, including toxicity. To mitigate such issues, it is proposed to replace the benzene fragment in the molecule, which is not involved in specific ligand-target interactions.
Saturated and conformationally rigid bicyclic molecules, such as 1,2- and 1,5-disubstituted bicyclo[2.1.1]hexanes and 2-oxabicyclo[2.1.1]hexanes, are suggested as bioisosteric replacements for ortho-disubstituted phenyl rings. These substitutions represent a promising strategy for obtaining new patentable molecules with improved bioactivity and physicochemical parameters.
Drop us a line if you have any questions: [email protected]