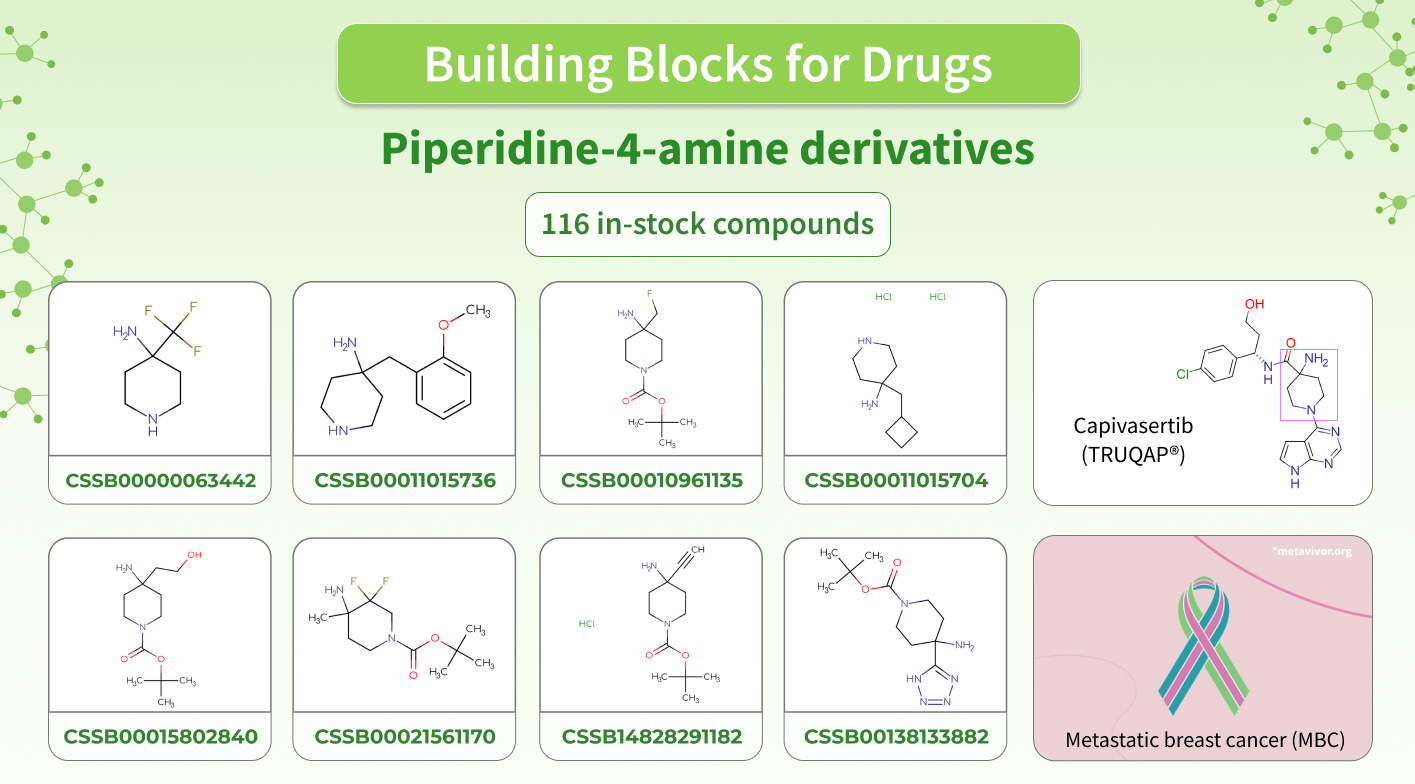
This November, Capivasertib (TRUQAP®) received approval by the FDA as a medicine for treating estrogen receptor (ER)-positive metastatic breast cancer (MBC) in adults.
It acts as an inhibitor of AKT1, AKT2, and AKT3 isoforms of serine/threonine kinase AKT (protein kinase B) that results in inhibiting the phosphorylation of substrates in the AKT signaling pathway. The AKT kinase plays a crucial role in regulating many processes including metabolism, cell survival, growth, and proliferation. Mutations and loss of functions in the AKT pathway are among the most common reasons for breast cancer.
Capivasertib contains piperidine-4-amine moiety in its backbone. To support your further research, we have made a set of Building Blocks that contain such cores, which could be a promising starting point in your research projects!