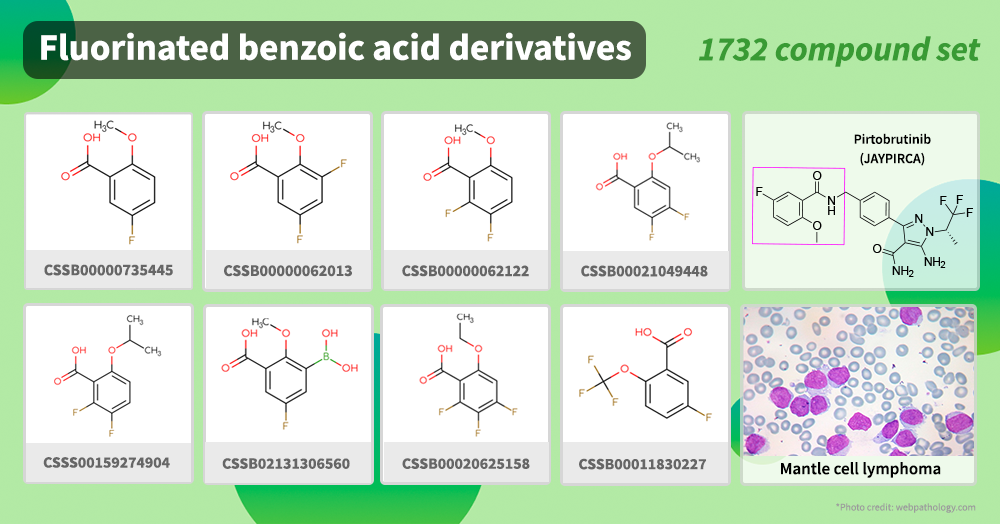
This January Pirtobrutinib (JAYPIRCA) received accelerated approval by the FDA as a drug for the treatment of refractory mantle cell lymphoma (MCL) in adults.
It is prescribed to patients who have had two lines of systemic therapy before. Pirtobrutinib is the first non-covalent Bruton's tyrosine kinase (BTK) inhibitor that is active against mutated targets. It contains fluorinated benzoic acid moiety in its backbone.