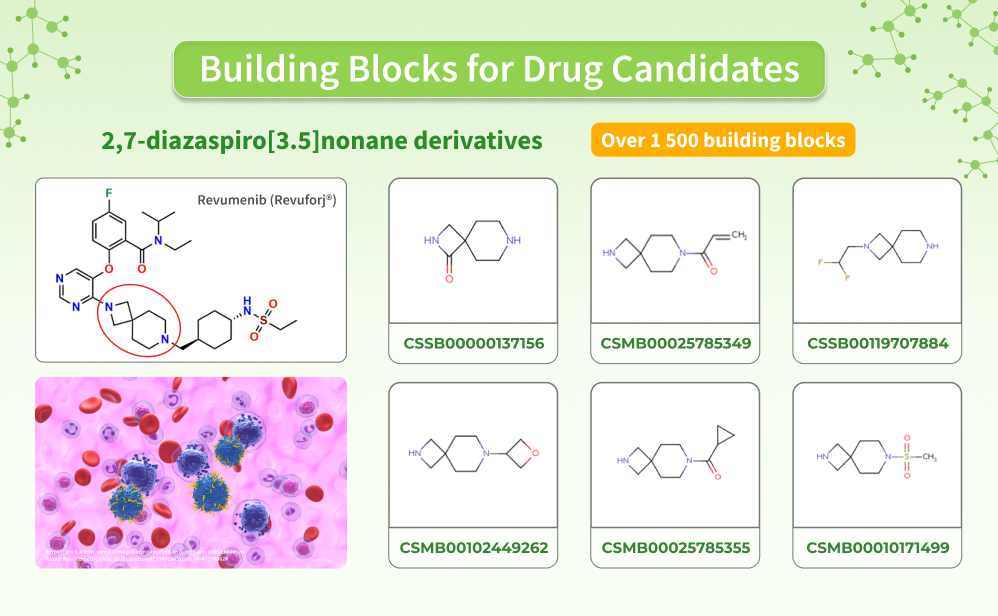
In November 2024, the FDA approved revumenib (Revuforj, Syndax Pharmaceuticals, Inc), the first-in-class menin inhibitor, for the treatment of relapsed/refractory acute leukemia with KMT2A translocation in patients aged 1 year and older.
Analyzing the structure of revumenib, a 2,7-diazaspiro[3.5]nonane fragment caught our attention. This unique moiety has inspired us to offer a set of building blocks for your drug discovery projects (https://chem-space.com/list/1386fe01d4ec4aa395ca7e89e1f5fa6e).
Even in an early-phase clinical trial, revumenib showed promising efficacy, inducing complete remission in about one-third of participants with acute myeloid leukemia. This drug prevents the interaction between menin and the rearranged KMT2A protein, commonly associated with aggressive forms of blood cancer. By targeting this pathway, revumenib effectively halts the growth of leukemic cells, providing a targeted therapeutic option for this challenging disease.
If you want to acquire building blocks or learn more about our offerings, please get in touch with us at [email protected].